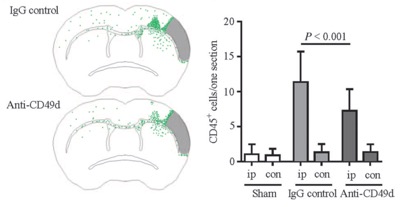
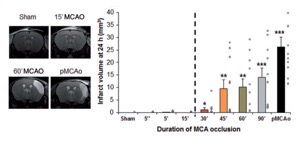
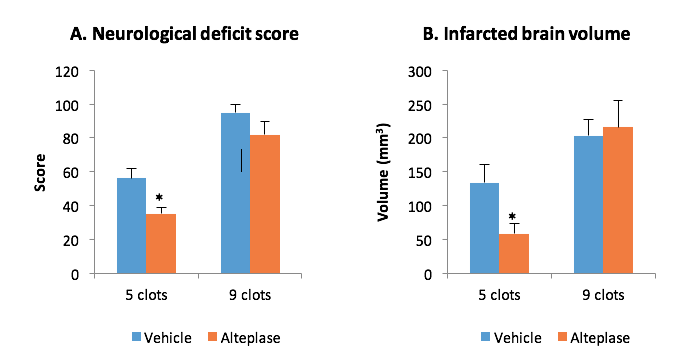
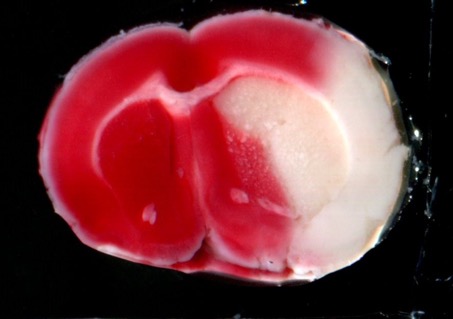
STROK@LLIANCE – Dedicated research Lab for Neurovascular diseases
Since 2017, STROK@LLIANCE has built strong expertise in preclinical research focused on neurovascular diseases.
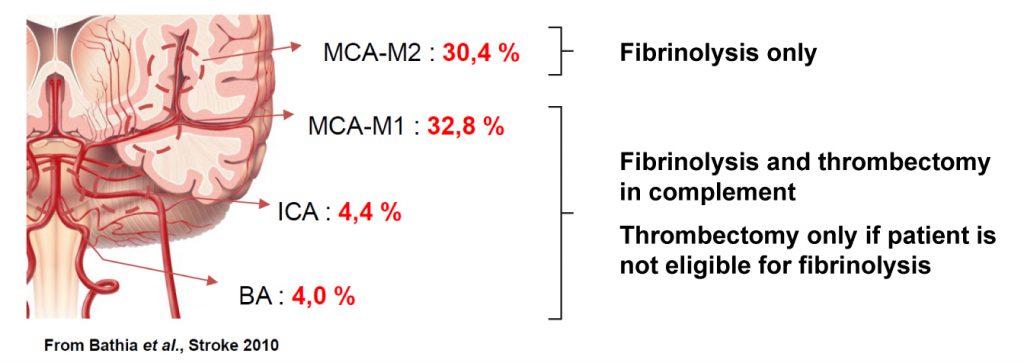
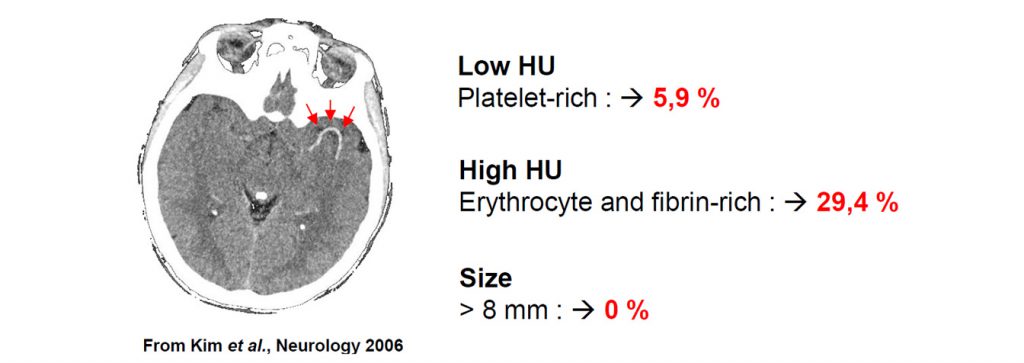
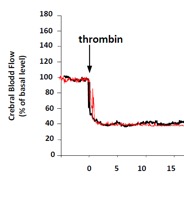
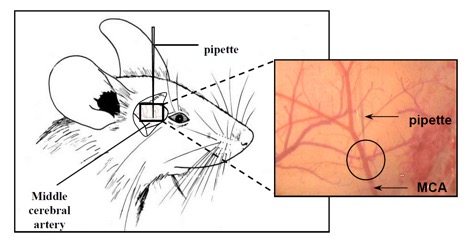
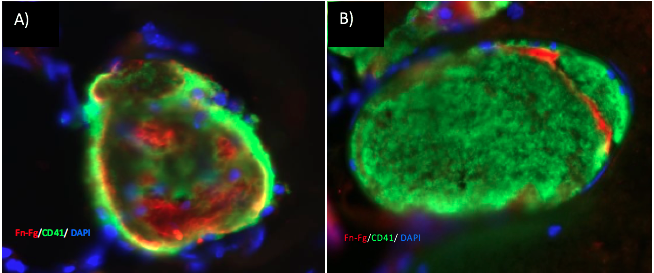
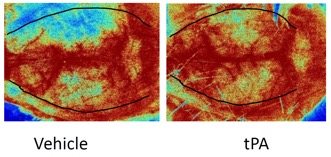
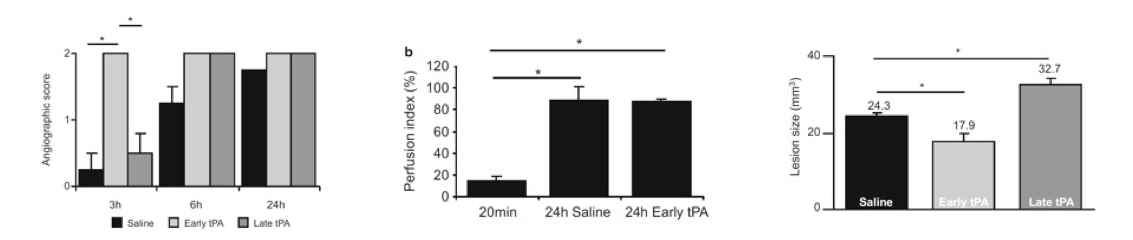
We offer high-translational efficacy and safety models that integrate key risk factors in an industrial standards environment. Our services cover both in vitro and in vivo approaches, supported by a comprehensive portfolio that includes hemostasis assays, cerebral hemorrhage and stroke models, and advanced imaging technologies such as MRI, PET, and molecular imaging. Our fully equipped platforms for bioassays, histology, and immunohistochemistry enable in-depth analysis and biomarker profiling. We offer both standardized and customized solutions tailored to your specific needs in the field of neurovascular research and stroke.