STROK@lliance – Expert Opinion #1
Annual Report of 2018
Strok@lliance is now certified ISO 9001 (2015 Edition)
Impact of Bradykinin Generation During Thrombolysis in Ischemic Stroke
Strok@lliance – Newsletter #4 -Back to the Strok@lliance Second Annual Meeting
Newsletter #3 – 2nd Strok@lliance annual meeting was a success!
Strok@lliance – Newsletter #2
JOIN US!
Strok@lliance is growing to provide its customers with high level and original services in preclinical stroke studies.
You have now the opportunity to join a young and dynamic team looking for challenge in a high level scientific and technologic environment !
Strok@lliance invites applications from eligible candidates for recruitment of a Scientific Laboratory Technician in stroke preclinical testing.
The company is looking for individuals who wants to contribute their skills to provide excellent work in a challenging and rewarding environment.
Find the application details here.
Focus on an innovative model of thromboembolic stroke reproducing the clinical situation
Recombinant tissue-type plasminogen activator (rt-PA) is the only approved drug treatment at the acute phase of ischemic stroke in the US and Europe. However, rt-PA has a short therapeutic window (4.5h after stroke onset), mainly because of the increased risk of hemorrhagic transformation from this time. Thus, only a few percentage of stroke patients will benefit from rt-PA-induced thrombolysis.
These last two decades, despite the efforts made to improve fibrinolysis or to develop neuroprotective drugs, all of them failed at the clinical stage. It is well admitted that experimental models of stroke are either too severe, not enough reproducible, or do not sufficiently mimic pathophysiology of the Human disease for a correct evaluation of drugs efficacy including neuroprotectants, thrombolytics alone or in combination.
In order to solve this problem, ESRP scientists developed a new translational model of thromboembolic stroke in mice, which overcomes several of these pitfalls. This model is now available for pharmacological studies with Strok@lliance.
In situ thromboembolic stroke is induced by the injection of purified thrombin in the Middle Cerebral Artery (MCA) at M1/M2 bifurcation, modelling a distal thrombosis eligible for thrombolytic treatments in humans. It results in reproducible ischemic lesions in the somatosensory cortex (≈20 mm3 ; Fig. 1). Moreover, it produces a significant strength deficit of the contralateral paws, lasting up to 14 days after stroke onset thus allowing functional recovery studies.
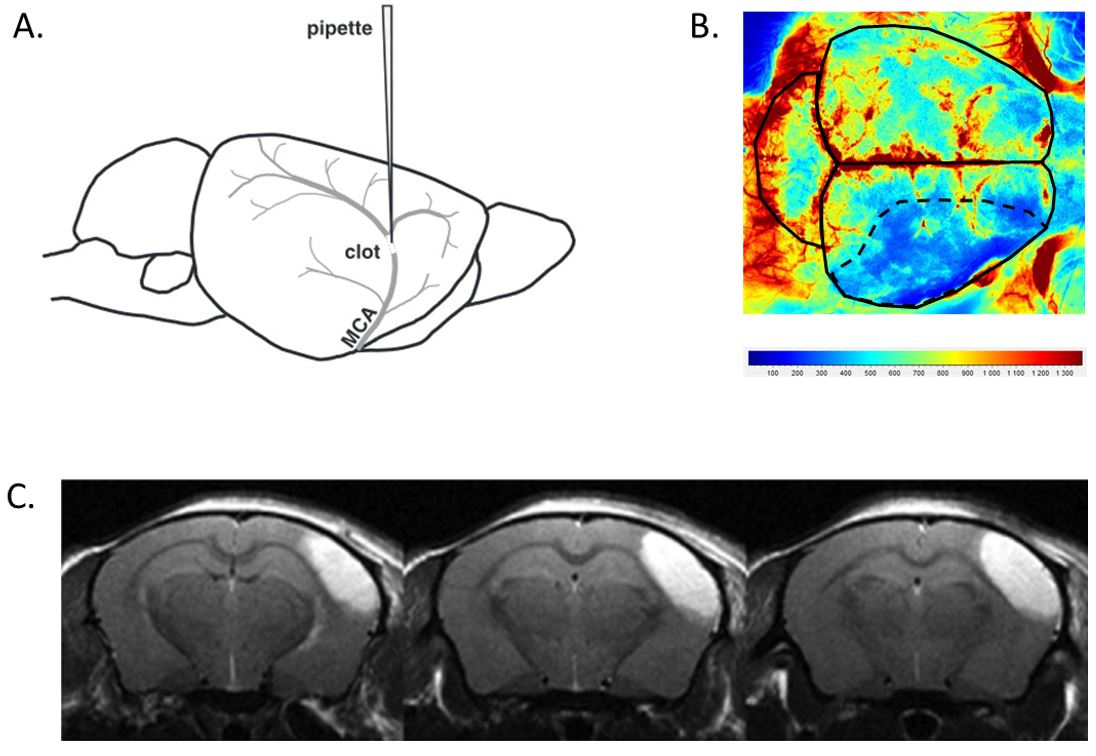 Figure 1. A. Thrombin injection in the MCA at M1/M2 bifurcation; B. Cerebral blood flow reduction in the ischemic hemisphere at stroke onset (laser speckle imaging); C. Representative lesion at 24h after stroke onset in saline-treated mice (T2* MRI).
Figure 1. A. Thrombin injection in the MCA at M1/M2 bifurcation; B. Cerebral blood flow reduction in the ischemic hemisphere at stroke onset (laser speckle imaging); C. Representative lesion at 24h after stroke onset in saline-treated mice (T2* MRI).
Due to its fibrin-rich content, the produced clot is sensitive to rt-PA induced thrombolysis. A retrospective data analysis from 26 preclinical studies performed with this model in 9 independent academic laboratories allowed to estimate the therapeutic windows of rt-PA on cerebral lesion volume. When started early after stroke onset, i.e. less than 40 min post-stroke, rt-PA treatment induces a reproducible reduction of the cerebral injury; treatment starting later than 90 min had no or deleterious effects. This study also confirmed the intra- and inter-laboratory reproducibility of this model (Fig. 2).
Thus, this model reproduces key features of human pathophysiology and pharmacology, while overcoming several pitfalls of classical models.
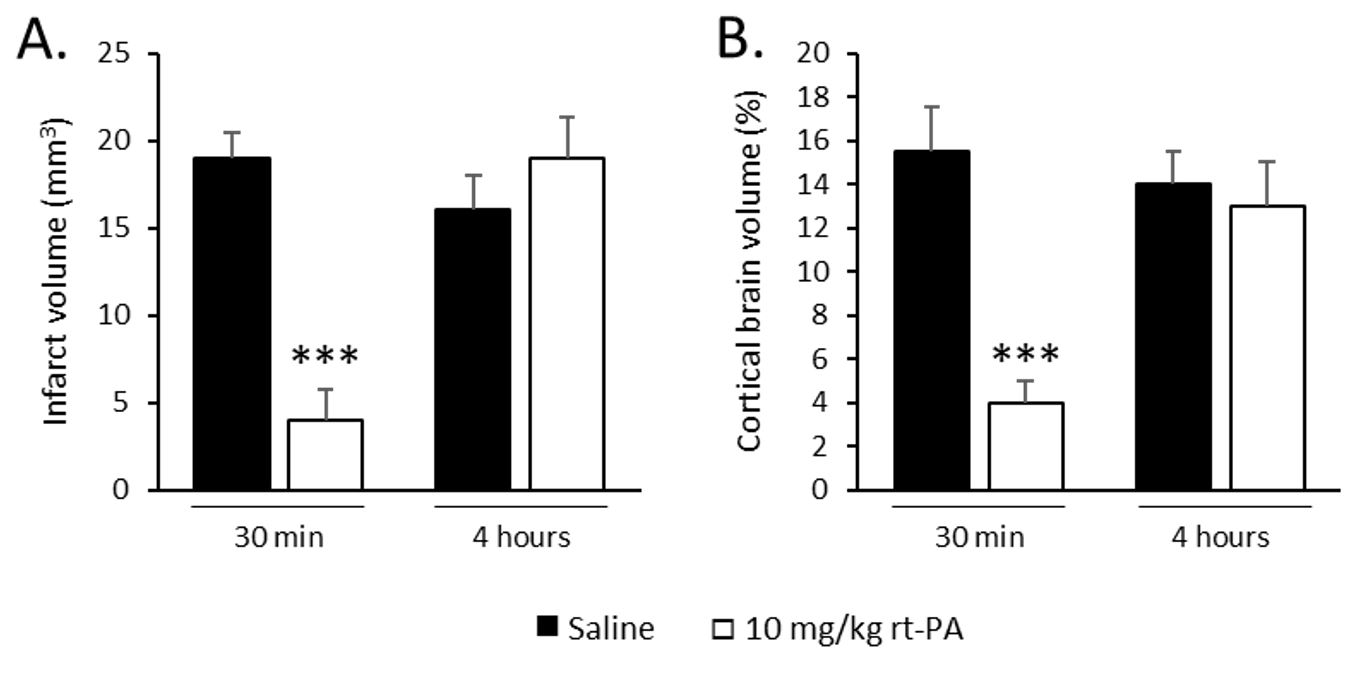
Figure 2. Effects of rt-PA i.v. perfused at 10 mg/kg when administered 30 min or 4 hours after stroke onset on: A. infarct volume and B. Brain oedema. Data are expressed as mean ± SEM. *** P < 0.001 versus saline.
References:
Orset C. et al. (2007) Mouse model of in situ thromboembolic stroke and reperfusion. Stroke 38:2771-8.
Macrez R. et al. (2011) Antibodies preventing the interaction of tissue-type plasminogen activator with N-methyl-D-aspartate receptors reduce stroke damages and extend the therapeutic window of thrombolysis. Stroke 42:2315-22.
El Amki M. et al. (2012) Experimental modeling of recombinant tissue plasminogen activator effects after ischemic stroke. Experimental neurology 238:138-44.


“Composed of highly skilled specialists, Strok@lliance is now able to manage projects for the pharmaceutical industry, ensuring that both expertise and creativity are brought into play in providing high-level animal studies in a quality-controlled environment. By taking full advantage of the CYCERON facilities, Strok@lliance accesses state-of-the-art technology and offers fine-tuned behavioral studies.”
N. Violle, Etap-Lab CEO, Strok@lliance Executive Partner
Meet us at ESOC 2017
Dr. Nicolas VIOLLE will attend to the 3rd European Stroke Organization Conference (ESOC) from 16-18 May, 2017 (Prague).
ESOC is one of the most exiting conference this year about clinical trials and developments in stroke management, treatment and prevention.
It will be the occasion for us to meet leaders from pharmaceutical industry to present STROM@LLIANCE, the new service 100% dedicated to preclinical stroke research.
Contact us from now to schedule a meeting.